
Healthcare Provider
For general questions or inquiries, or to order materials please contact us at:
Prescribing and Protecting with DAW-1 is Now Easier
- SUBVENITE Starter Kits are OWP Pharmaceuticals’ branded generic alternative (AB rated) to Lamictal® (lamotrigine) tablets Starter Kits* [click here to learn more about generic bioequivalence]
- Writing DAW-1 (Dispense As Written — or appropriate language required by your state) ensures that your patient’s prescription is not substituted for another generic.
- Your EHR system may allow you to attach the SUBVENITE Patient Savings Card and send it with the e-prescription directly to the pharmacy.

Orange Starter Kit: Total 49 Tablets
(42 X 25mg) (7 X 100mg)
NDC 69102-300-01

Blue Starter Kit: Total 35 Tablets
(35 X 25mg)
NDC 69102-306-01

Green Starter Kit: Total 98 Tablets
(84 X 25mg) (14 X 100mg)
NDC 69102-312-01
Why Start with SUBVENITE Starter Kits?
- SUBVENITE Starter Kits help simplify treatment initiation for you and your patients in a more structured way than is offered by multiple weekly prescriptions and a bottle of 25 mg tablets

- Each Kit is based on FDA-approved 5-week titration schedules for patients initiating lamotrigine therapy
- You only need to write one prescription for a 5-week Starter Kit
- Each Kit comes with a week-by-week blister pack with perforated daily dosing for on-the-go use
- With the Savings Card all patients can obtain the SUBVENITE Starter Kit at a significantly lower cost than Lamictal®*†1
4 Easy Steps to Start with the SUBVENITE Starter Kit


Determine which 5-week titration schedule is appropriate for your patient
(Orange, Blue, or Green)


Write or e-Prescribe SUBVENITE Starter Kit (Orange, Blue, or Green) DAW-1


Provide savings card to patient or direct patient to access the savings card on a mobile device at subvenite.com


Tell your patient to pick up the SUBVENITE Starter Kit from the pharmacy and to contact OWP Pharmaceuticals if there are questions
Assistance at the Pharmacy
Inform your patient that if they have difficulty filling their prescription at their local pharmacy they can contact OWP Pharmaceuticals for assistance at
1-800-273-6729
They can also call this number if they have any questions regarding their SUBVENITE Starter Kit prescription
A portion of the profits from each SUBVENITE Starter Kit prescription helps treat people with neurological disorders in under-resourced areas globally
Please visit www.rowglobal.org to learn more
What is Generic Bioequivalence?
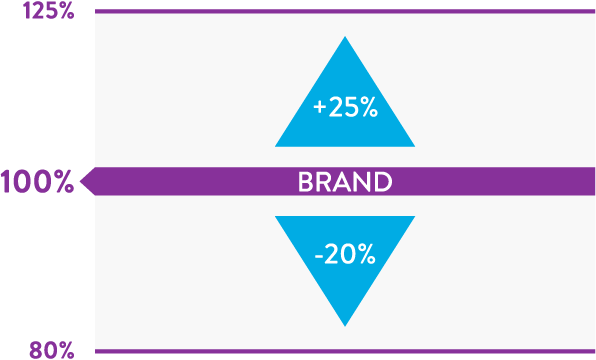
Generic products are considered bioequivalent to the brand (reference drug) if the confidence interval (90%) for AUC† and Cmax† is within 80-125% of the brand (reference drug).2
The FDA has determined that generic drugs that are classified as therapeutically equivalent can be substituted with the full expectation that the substituted product will produce the same clinical effect and safety profile as the reference product.3
FDA Approved Range for Generic Bioequivalency
References
1. https://www.goodrx.com/lamictal?dosage=49-tablets-of-25mg-and-100mg-orange&form=kit&label_override=Lamictal&quantity=1
2. Center for Drug Evaluation and Research. Guidance for Industry: Statistical Approaches to Establishing Bioequivalence. Silver Spring, MD: Food and Drug Administration, US Department of Health and Human Services; 2001. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070244.pdf. Accessed September 26, 2013.
3. Office of Generic Drugs, Center for Drug Evaluation and Research. Approved Drug Products With Therapeutic Equivalence Evaluations. 33rd ed. Silver Spring, MD: Food and Drug Administration, US Department of Health and Human Services; 2013. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/ucm071436.pdf. Accessed September 26, 2013.
*Lamictal® is a registered trademark of GlaxoSmithKline, Inc.
†Terms and conditions apply; see subvenite.com/savings-program
For general questions or inquiries please contact us at: info@owppharma.com
For SUBVENITE Starter Kit questions please contact us at: medinfo@owppharma.com
To report-safety related concerns please contact us at: safety@owppharma.com
OWOS1063V6 05/23
SUBVENITE SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS SKIN RASHES
Cases of life-threatening serious rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis, and/or rash-related death have been caused by lamotrigine. The rate of serious rash is greater in pediatric patients than in adults. Additional factors that may increase the risk of rash include:
- coadministration with valproate.
- exceeding recommended initial dose of SUBVENITE.
- exceeding recommended dose escalation of SUBVENITE.
Benign rashes are also caused by lamotrigine; however, it is not possible to predict which rashes will prove to be serious or life threatening. SUBVENITE should be discontinued at the first sign of rash, unless the rash is clearly not drug related.
Please refer to the full Prescribing Information for SUBVENITE (lamotrigine tablets, USP) for the complete boxed warning.
SUBVENITE (lamotrigine tablets, USP) are indicated for:
Epilepsy—adjunctive therapy in patients aged 2 years and older:
- partial-onset seizures.
- primary generalized tonic-clonic seizures.
- generalized seizures of Lennox-Gastaut syndrome.
Epilepsy—monotherapy in patients aged 16 years and older: Conversion to monotherapy in patients with partial-onset seizures who are receiving treatment with carbamazepine, phenytoin, phenobarbital, primidone, or valproate as the single antiepileptic drug.
Bipolar disorder: Maintenance treatment of bipolar I disorder to delay the time to occurrence of mood episodes in patients treated for acute mood episodes with standard therapy.
LIMITATIONS OF USE
Treatment of acute manic or mixed episodes is not recommended. Effectiveness of SUBVENITE in the acute treatment of mood episodes has not been established.
CONTRAINDICATIONS
SUBVENITE is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients.
WARNINGS & PRECAUTIONS
- Cases of life‐threatening serious rashes, including Stevens‐Johnson syndrome, toxic epidermal necrolysis, and/or rash‐related death, have been caused by SUBVENITE. The rate of serious rash is greater in pediatric patients than in adults. Refer to SUBVENITE – BOXED WARNING.
- SUBVENITE should not be abruptly discontinued. In patients with epilepsy there is a possibility of increasing seizure frequency. Unless safety concerns require a more rapid withdrawal, the dose of SUBVENITE should be tapered over a period of at least 2 weeks. Refer to SUBVENITE – DOSAGE AND ADMINISTRATION.
- Hemophagocytic lymphohistiocytosis (HLH) has occurred in adult and pediatric patients taking SUBVENITE. HLH is a life-threatening syndrome of pathologic immune activation characterized by clinical signs and symptoms of extreme systemic fibrosis. It is associated with high mortality rates if not recognized early and treated. HLH has presented with systemic inflammation (fever, rash, hepatosplenomegaly, and organ system dysfunction) and blood dyscrasias. Symptoms have been reported to occur within 8 to 24 days following the initiation of treatment. Patients who develop early manifestations of pathologic immune activation should be evaluated immediately, and a diagnosis of HLH should be considered.
- Multiorgan hypersensitivity reactions, also known as Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), have occurred with SUBVENITE. Some have been fatal or life threatening. DRESS typically, although not exclusively, presents with fever, rash, and/or lymphadenopathy in association with other organ system involvement, such as hepatitis, nephritis, hematologic abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection. Eosinophilia is often present. This disorder is variable in its expression, and other organ systems not noted here may be involved. Fatalities associated with acute multiorgan failure and various degrees of hepatic failure have been reported. Prior to initiation of treatment with SUBVENITE, the patient should be instructed that a rash or other signs or symptoms of hypersensitivity (e.g., fever, lymphadenopathy) may herald a serious medical event and that the patient should report any such occurrence to a healthcare provider immediately.
- In vitro testing showed that SUBVENITE exhibits Class IB antiarrhythmic activity at therapeutically relevant concentrations. Based on these in vitro findings, SUBVENITE could slow ventricular conduction (widen QRS) and induce proarrhythmia, which can lead to sudden death, in patients with clinically important structural or functional heart disease (i.e., patients with heart failure, valvular heart disease, congenital heart disease, conduction system disease, ventricular arrhythmias, cardiac channelopathies [e.g., Brugada syndrome], clinically important ischemic heart disease, or multiple risk factors for coronary artery disease). Any expected or observed benefit of SUBVENITE in an individual patient with clinically important structural or functional heart disease must be carefully weighed against the risks for serious arrythmias and/or death for that patient. Concomitant use of other sodium channel blockers may further increase the risk of proarrhythmia.
- There have been reports of blood dyscrasias that may or may not be associated with multiorgan hypersensitivity (also known as DRESS). These have included neutropenia, leukopenia, anemia, thrombocytopenia, pancytopenia, and, rarely, aplastic anemia and pure red cell aplasia.
- Antiepileptic drugs (AEDs), including SUBVENITE, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Anyone prescribing SUBVENITE must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment with SUBVENITE, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated. Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, the emergence of suicidal thoughts, behavior, or thoughts about self harm. Behaviors of concern should be reported immediately to healthcare providers.
- Therapy with SUBVENITE increases the risk of developing aseptic meningitis. Because of the potential for serious outcomes of untreated meningitis due to other causes, patients should also be evaluated for other causes of meningitis and treated as appropriate.
- Medication errors involving SUBVENITE have occurred. In particular, the name SUBVENITE or lamotrigine can be confused with the names of other commonly used medications. Medication errors may also occur between the different formulations of SUBVENITE.
- Some estrogen containing oral contraceptives have been shown to decrease serum concentrations of lamotrigine. Dosage adjustments will be necessary in most patients who start or stop estrogen containing oral contraceptives while taking SUBVENITE.
- Valid estimates of the incidence of treatment emergent status epilepticus among patients treated with SUBVENITE are difficult to obtain because reporters participating in clinical trials did not all employ identical rules for identifying cases. At a minimum, 7 of 2,343 adult patients had episodes that could unequivocally be described as status epilepticus. In addition, a number of reports of variably defined episodes of seizure exacerbation (e.g., seizure clusters, seizure flurries) were made.
- During the premarketing development of SUBVENITE, 20 sudden and unexplained deaths were recorded among a cohort of 4,700 patients with epilepsy (5,747 patient years of exposure).
- Because valproate reduces the clearance of lamotrigine, the dosage of SUBVENITE in the presence of valproate is less than half of that required in its absence.
- Because lamotrigine binds to melanin, it could accumulate in melanin rich tissues over time. This raises the possibility that lamotrigine may cause toxicity in these tissues after extended use. Although there are no specific recommendations for periodic ophthalmological monitoring, prescribers should be aware of the possibility of long-term ophthalmologic effects.
- There have been reports of decreased lamotrigine concentrations during pregnancy and restoration of pre partum concentrations after delivery. As with other AEDs, physiological changes during pregnancy may affect lamotrigine concentrations and/or therapeutic effect. Dosage adjustments may be necessary to maintain clinical response.
- Lamotrigine is present in milk from lactating women taking SUBVENITE. Caution should be exercised when SUBVENITE is administered to a nursing woman.
- Safety and efficacy of SUBVENITE used as adjunctive treatment for partial-onset seizures in very young pediatric patients (aged 1 to 24 months) has not been demonstrated.
- Safety and efficacy of SUBVENITE for the maintenance treatment of bipolar disorder in pediatric patients has not been demonstrated.
- Because lamotrigine is metabolized predominantly by glucuronic acid conjugation, drugs that are known to induce or inhibit glucuronidation may affect the apparent clearance of lamotrigine and doses of SUBVENITE may require adjustment based on clinical response.
DOSING GUIDELINES & CONSIDERATIONS
- Refer to the SUBVENITE – DOSAGE AND ADMINISTRATION section of the full prescribing information for recommended dosing guidelines for SUBVENITE.
- There are a number of significant drug interactions with SUBVENITE. Refer to the SUBVENITE – DRUG INTERACTIONS section of the full prescribing information.
ADVERSE REACTIONS
- See the ADVERSE REACTIONS section of the SUBVENITE full prescribing information for adverse reaction rates from clinical trials conducted under widely varying conditions.
- The most common adverse reactions (incidence ≥10%) in adults were dizziness, headache, diplopia, ataxia, nausea, blurred vision, somnolence, rhinitis, pharyngitis, and rash. Additional adverse reactions (incidence ≥10%) reported in children included vomiting, infection, fever, accidental injury, diarrhea, abdominal pain, and tremor.
- The most common adverse reactions (incidence >5%) in adults with bipolar disorder were nausea, insomnia, somnolence, back pain, fatigue, rash, rhinitis, abdominal pain, and xerostomia.
- To report SUSPECTED ADVERSE REACTIONS, contact OWP Pharmaceuticals Inc. at 1-800-273-6729 or www.fda.gov/medwatch.
Please refer to the full Prescribing Information for SUBVENITE Tablets, including Medication Guide at www.subvenite.com.
02/2023
